
Image of Alberta oil sands magnified 10x.
A new process to extract hydrocarbons, such as oil and bitumen, from sand and other mineral matter could make some energy resources cleaner.
Canada is now the largest supplier of oil to the United States, the size of its reserves second only to Saudi Arabia. About half of that oil is derived from Canadian tar sands, referred to as “the world’s dirtiest oil” because of the significant environmental impact associated with extracting and upgrading the bitumen.
There are estimated to be about 32 billion barrels of oil in tar sands in the western United States, mainly in Utah. However, because they are located in a desert region, a technology that does not need large quantities of water and does not produce wastewater is needed in order to extract the oil.
Hydraulic fracturing and other new technologies have opened the door to vast untapped gas and oil reserves in formations such as the Marcellus and Bakken shale. In drilling the wells to access these reservoirs, 300-1,200 tons of sand, shale, or other minerals mixed with oil are brought to the surface and dumped in landfills.
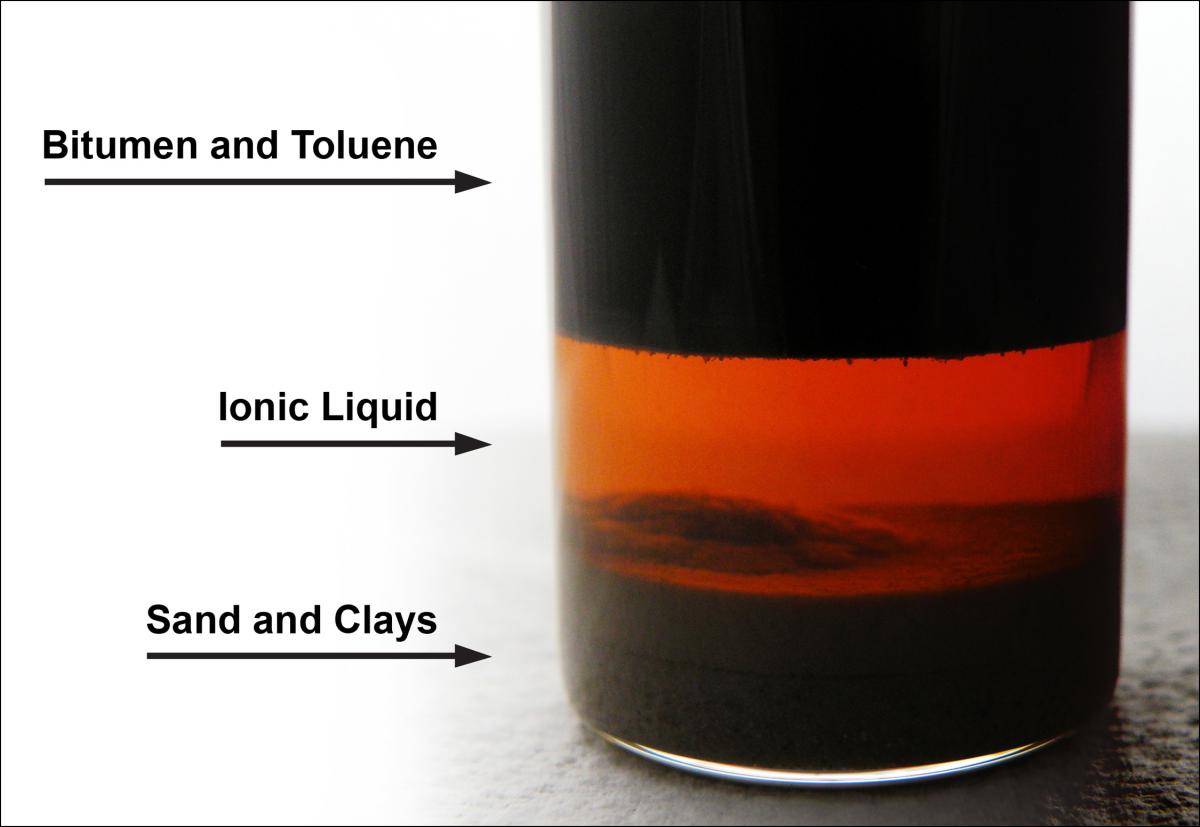
Layers after separation process using ILs.
Developing a cleaner process
The separation and extraction of oil and bitumen from soil, sand, or other forms of mineral matter, are difficult processes and current technologies are much more energy and resource intensive than those for conventional oil wells. In addition, the disposal of waste products poses further problems. For example, the separation of bitumen from tar or oil sands requires large amounts of water that then becomes toxic to aquatic life, a major environmental and cost problem.
A team of researchers at Penn State, led by Dr. Paul Painter, professor of polymer science and engineering, has developed a novel method for separating hydrocarbons from mineral matter through the use of ionic liquids (ILs). ILs are similar to common table salt except they are liquids below 100°C (by comparison table salt, NaCl, melts at about 800°C). They have powerful solvent properties, outstanding chemical and thermal stability, a very low degree of flammability, and almost negligible vapor pressure.


Aron Lupinsky, research assistant, EMS Energy Institute, works with the demonstration unit.
Extracting hydrocarbons using ILs is simple and relatively inexpensive. The bitumen, solvent, and sand/clay mixture are separated into three distinct layers and each is easily recoverable. The separation process occurs at room temperature and requires very little water. As a result, minimal amounts of energy are used and the need to import large quantities of water for processing oil or tar sands is essentially eliminated.
Painter and his team, comprised of personnel from the EMS Energy Institute, including Bruce Miller, senior scientist; Aron Lupinsky, research assistant; Dr. Maria Sobkowiak, research associate; and Andrea Choperna, research assistant, are conducting laboratory- and demonstration-scale work. The team constructed a demonstration unit consisting of a 200-gallon mixing tank, two polyethylene tanks for gravity separation of coarse sand from liquids, and a coalescing unit to separate oil or diluted bitumen from the ILs. The ILs are then recirculated to the main tank for further separation. Researchers are now installing a centrifuge to accelerate and improve the oil/IL separation.
With this new method, researchers have completely recovered bitumen, even from low-grade tar sands, and crude oil from a sand/oil mixture. The resulting hydrocarbon is free of particle fines and shows no contamination from the ILs. Residual sands and clays are also very clean and can be returned to beaches, used in the remediation of environmental scars from mining, or processed into a value-added product.
Residual IL is removed from the sand using a water rinse. The water and ILs are separated with vacuum distillation and both are recycled. The toxic wastewater, currently stored in vast tailing ponds, becomes nonexistent and recycling the ILs yields significant savings, as much as 50 percent when compared to current technologies.
Reducing the impact of energy resources
One area where ILs can make a considerable impact is in the mining of oil sands where bitumen is either dug from open pit mines or, if too deep to be strip-mined, obtained using in-situ extraction methods, such as steam-assisted gravity drainage (SAGD). The bitumen is often contaminated with minerals, mainly clays, and these oil-contaminated waste solids are ultimately sent to landfills.
The United States was importing over 2 million barrels per day of crude oil from Canada in January 2011, according to the U.S. Energy Information Administration, and nearly all of that oil was produced by upgrading bitumen extracted from oil or tar sands. In addition, there are estimated to be about 32 billion barrels of oil in tar sands in the western United States, mainly in the desert region of Utah where there are no commercial scale operations.
Already, the research team has successfully separated oil from minerals in oily wastes from SAGD operations and materials from Canadian tailing ponds, resulting in increased oil recovery and the elimination of toxic tailing ponds, significantly lessening the impact of what is currently called “the World’s dirtiest oil.” Since ILs require very little water, they also make extracting oil in the Utah desert regions feasible.
In addition, ILs could improve the process for recovering heavy oil, which is found in about thirty countries, with the largest deposit in Venezuela. Instead of expensive thermal extraction methods such as steam injection, a new technique called cold heavy oil production with sand (CHOPS) can significantly increase well productivity. However, CHOPS involves the continuous production of sand along with oil, presenting separation and disposal problems. The research team has successfully recovered heavy oil from deposits within the United States.
ILs could also play an important role in reducing waste from natural gas drilling operations. While hydraulic fracturing in formations such as the Marcellus and Bakken shale has allowed the recent exploitation of previously inaccessible gas and oil reserves, tremendous amounts of waste drill cuttings, the sand, shale, or other minerals brought to the surface during drilling, must now be shipped to landfills. A typical horizontal Marcellus shale well produces 300-1200 tons of cuttings. Each drilling site or pad can contain eight to twelve wells. In addition to the expense, up to $150 per ton, to dispose of these cuttings, they also contain large amounts of oil, which creates compaction problems for landfills.
ILs can be used to separate oil from contaminated cuttings. The recovered oil, which can be as much as 20 percent of the weight of the drill cuttings, can be recycled to drilling muds or used as a fuel, while the cleaned sand and soil can be used for land reclamation, soil amendments, easily compacted landfill cover, or as a starting material in making a value-added product, proppants (a material sent down with the fracking fluid to prop open cracks in the rock).
For more information contact Dr. Painter, painter@ems.psu.edu, or Mr. Miller, bgm3@psu.edu.
Other applications under investigation
|

